Developing the
Next Generation of
Cancer Immunotherapies
Universal Ready-to-Use
Tumor Infiltrating Lymphocytes
Tumor infiltrating lymphocytes (TILs) represent a heterogeneous population of lymphocytes that naturally infiltrate solid tumors.
In TIL-based adoptive cellular therapy (ACT), naturally occurring TILs are isolated from a tumor biopsy and then expanded to large numbers ex vivo in the lab before being given back to the patient to fight the cancer. ACT is a form of immunotherapy that is rapidly growing in clinical investigation because of its ability to augment the number, specificity, and reactivity of T cells against cancer cells.
However, many challenges hinder TIL-based ACT clinical applications. Universal ready-to-use T cells (UR-TIL) which could be broadly given to any patient, prepared in advance, and readily available would be clinically ideal and more cost-effective.
Why We’re Different
Universal Ready-to-Use TILs
No resectable tumor? No problem.
Need consistent quality? We’ve got it under control.
Not enough immune cells? Unlimited amount of Tils.
Worried about safety such as GVHD? Worry no more.
Suppression of TME? Optimized Ex Vivo culture.
Solid tumors? We can treat those too.
Producible at scale? Been there, done that.
Affordable? Of course.
The Advantages of UR-TIL
How Our Technology Works
The Status Quo
The Challenge
Our Advantage
✅ A resectable lesion from a tumor is not necessary
⚠️ Such a lesion is not always and sometimes not often available e.g. patients with pancreatic or brain tumors where surgery isn’t possible
⚫ A resectable lesion from the tumor is necessary
✅ We can culture tumor and immune effector cells under standard, clean, and controllable conditions
⚠️ Those bacteria make the culture process prone to failure
⚫ Some tumors contain a large number of bacteria e.g. gastrointestinal tumors
✅ We can induce effector cells in vitro to avoid the immunosupression of the tumor host
⚠️ This can happen for various reasons such as the tumor microenvironment and inhibitory function of PD-1
⚫ The function of the immune cells can be immunosuppressed
✅ Our preparation process works independently of the patient’s clinical condition ensuring its efficacy for patients of all kinds
⚠️ For such patients the quality of the immune cells cannot be guaranteed
⚫ Many patients are undergoing chemotherapy and/or radiotherapy
✅ Our technology allows for the production of effectively unlimited numbers of cells, allowing for usage in active intensive treatment as well as long-term consolidation treatment
⚠️ Fewer immune cells means less treatment options and ultimately a less effective treatment
⚫ The quantity of the immune cells produced is limited
✅ We can and have treated patients with many types of cancer including but not limited to liver, bowel, lung, stomach, breast, ovarian, and pancreatic cancer
⚠️ The clinical applicability of these treatments is significantly limited
⚫ Given the above restrictions not many tumor types can be treated
✅ Our products are ready-to-use and can be administered immediately without any need for delays
⚠️ Prompt treatment is essential for patients with cancer
⚫ Culturing these cells can take a month or more
✅ Our products can be manufactured at scale and used for virtually any patient, any tumor, anywhere
⚠️ This simultaneously drives up cost and hinders quality control
⚫ This treatment requires extensive customization
What We Can Achieve

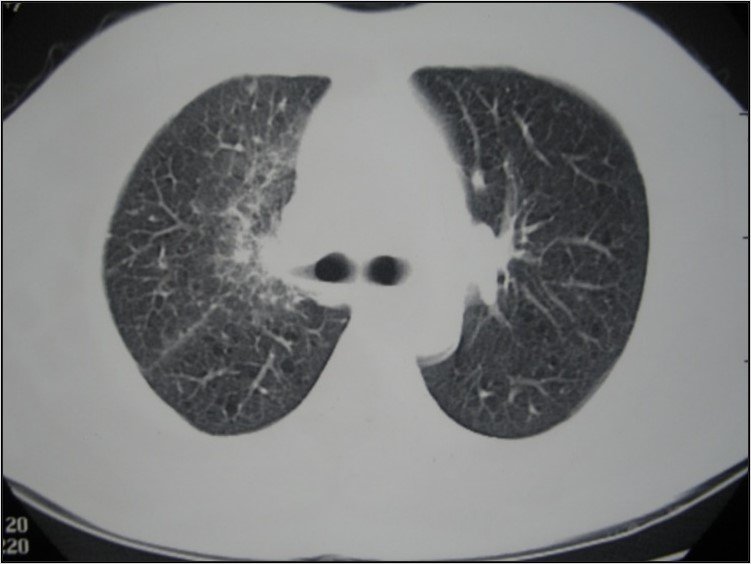
We help cancer patients with UR-TIL
A picture tells a thousand words, but these pictures tell more than that. We’ve successfully treated hundreds of cancer patients—improving quality of life and increase survive time with minimal side effects.
The pictures above are: a melanoma patient receiving UR-TIL monotherapy; a pancreatic cancer patient with ascites receiving our UR-TIL monotherapy; and a NSCLC patient with pleural effusion receiving our TIL therapy in addition to targeted medicine.
What we’ve done
Treated patients with solid tumors including liver, lung, breast, ovarian, and pancreatic cancer
Tackled tough cases with patients with advanced tumors including pleural effusion and ascites
Achieved successful treatment outcomes without GVHD, CRS, or autoimmune reactions
Improved clinical symptoms significantly including tumor shrinkage, tumor marker decline, and ultimately increase survival time
Stimulated cellular and humoral immune function
Achieved large number of activated lymphocytes in the tumor tissue of the patients after treatment
Universal Ready-to-Use Cytotoxic T lymphocytes (CTL) for the Backbone of CAR-T or TCR-T Cells Manufacture
The CAR-T products currently approved are derived from autologous T cells and must be generated on a custom basis.
Autologous CAR-T cells are manufactured by taking T cells from an individual patient, the whole manufacturing process requires multiple steps, such as a blood draw, isolation, activation, transfection, expansion, formulation, release, cryopreservation, transport, and administration.
Therefore, CAR-T cell therapy is a time and labor-consuming process and has an inherently high risk of batch failure. The vein-to-vein time (the process of collecting the T-cells to treat the patient with CAR-T) currently can take as much as eight weeks, twice as long as it took in 2020.
This rigid design is also associated with extremely high manufacturing costs that limit the clinical availability to the patients. For these reasons, autologous T-cell production platforms remain a critical limiting factor for large-scale clinical applications.
Increased patient demand, manufacturing capacity limitations and extensive wait times as the three biggest challenges keeping many eligible patients from receiving treatment.
1. Previous cytotoxic therapies can negatively affect the efficacy and durability of resultant CAR products.
2. Critical quality attributes (CQAs) such as cell number, viability, purity and CAR expression will be highly variable from batch to batch.
3. Long-term ex vivo expansion can result in T-cell exhaustion which reduces the effector function.
4. The resulting product heterogeneity and lack of reference standards complicate the evaluation of safety and efficacy.
5. There is also a high batch failure rate during the manufacturing process.
6. Time and labor-consuming manufacturing process.
7. Limited manufacturing capacity and Lack of qualified and trained staff with the skills required to produce these customized treatments.
8. Extremely high manufacturing and product quality control costs.
Challenges facing autologous CAR-T therapy
Allogeneic CAR Strategy by Using UR-CTL
Unlike autologous cell therapy, allogenic ready-to-use CAR-T cells obtain the starting T cells from healthy donors. The quality of the production process is more controllable, the culture results are also more efficient and reliable, and the immune cells can be manufactured on a large-scale and batch mass-produced for the treatment of a group of patients.
It should be emphasized that the immune cells we developed are mainly CD8+ CTLs. Analysis of the immune cell phenotypic subsets by flow cytometry showed that the CD8+ T lymphocytes we obtained reached over 95%. Since the current CAR-T products are manufactured mainly based upon CD8+CTL, we propose that our universal ready-to-use CTL be used for the Backbone of CAR-T or TCR-T Cells Manufacture of universal Car-T (UCAR-T) cells and universal TCR-T (UTCR-T) cells as well.
Advantages of Allogeneic UCAR-T:
As a new medical technology, the UR-CTL or UR-TIL (allogeneic) immune cells have been used for the treatment of solid tumors as well as hematological malignancies. The results of clinical applications of this UR-CTL or UR-TIL indicate that it is safe.
An excellent clinical safety profile was observed by the multi-clinical center’s application, with only moderate fever observed in a few patients. No CRS or GVHD nor autoimmune diseases were observed.
Clinical responses include improving the life of quality, increasing survival time, and inducing tumor regression associated with decreased or normalization of tumor biomarkers. These results indicate that the adoptive transferred UR-CTL is able to exert graft versus leukemia (GVL) or graft versus tumor (GVT) but avoid GVHD.
Our preliminary results suggest that after CAR transduction, the cytotoxicity of CTL is increased. Compared with CAR-T, CTL-CAR have slightly higher cytotoxicity, and the cytotoxicity is dose-dependent (Figure Left). When target cells are changed to K562, compared with CAR-T, both CTL and CTL-CAR have higher cytotoxicity, and the cytotoxicity is also dose-dependent (Figure Right).
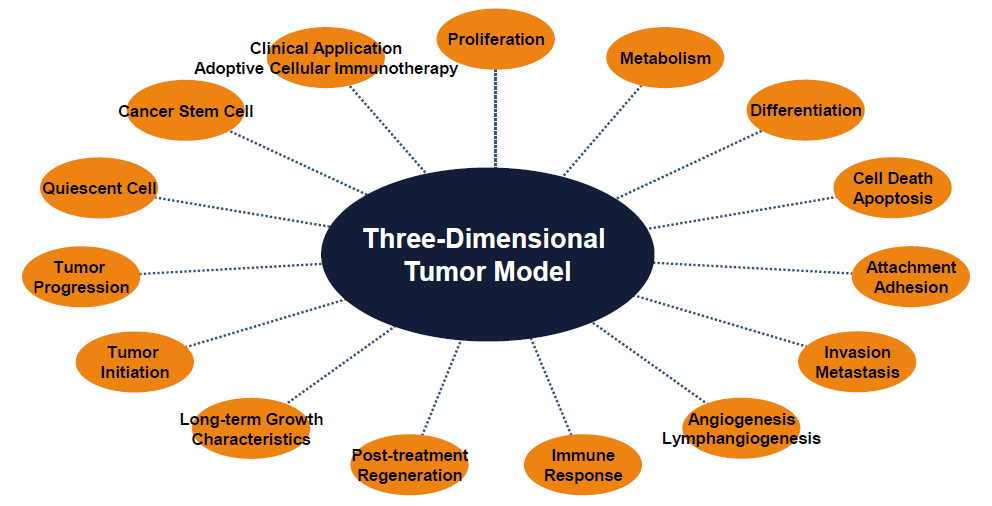
Our 3D Culture Technology &
Solid Tumor Models
Tumor cells in this system can form tumors at an early stage (48-72 hours) and can also grow long-term in vitro for up to several months. The other features of our 3D culture technology include transparency and dynamic observation of the tumor cells at any time. These advantages make the 3D cell culture system very suitable for the observation of the multi-stage, multi-step, complex process of tumor formation and development.
We provide a 3D space suitable for tumor cell movement and growth to form cancers and microcarcinomas similar to those in the body. Malignant tumor cells can form tumors of different sizes in this 3D culture system. The small tumor model formed by tumor cells in this culture system can be used for early diagnosis and treatment of tumors.
These in vitro tumor models provide a unique environment for the study of cancer to perform various tumor-related clinical studies. Thus, these 3D tumor models provide a valuable tool for tumor prevention, auxiliary diagnosis, improved treatment and evaluation of prognosis.
Advantages of a 3D Tumor Model
What Can Be Done - 3D Tumor Model
3D Culture Liver Cancer Cells
3D Culture Colon Cancer Cells
Other Related
Technologies and Products
3D Tumor Model for Cancer Vaccine
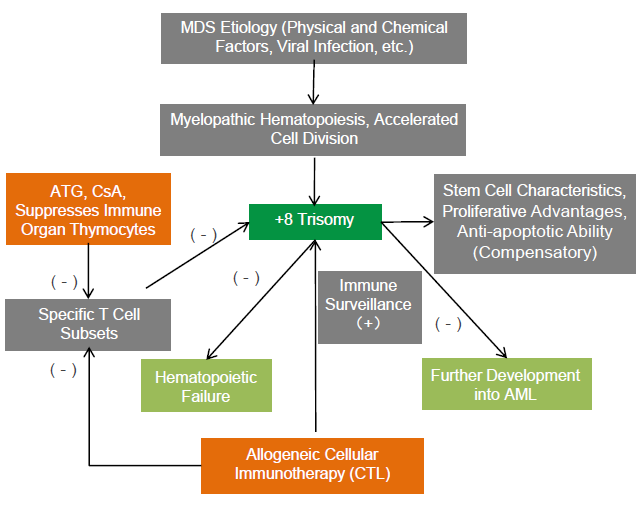
Allogeneic Immune Cell Therapy for Patient with MDS
Partnership Opportunities
Currently, the Xinghua team is looking for partners to jointly develop allogeneic universal ready-to-use immune cells (UR-TILs) and produce backbone cells for other types of universal immune cell preparations such as UCAR-T, UTCR-T cells, or other types of immune cells used for adoptive immune cell therapy.
We aim to improve the quality of immune cells, reduce the cost associated with treating these diseases, improve the safety of cell therapy, and ultimately better serve those patients suffering from these devasting and deadly diseases through our technologies.
We look forward to exploring the ways we can work together to achieve this mission.